Albuquerque hospitals at 140% capacity
And it is NOT from Covid
This short article is a respite from my long diatribes, and is a continuation of my post “Hospitals are full of vaccinated patients”.
I came across this news item from ABC News, “Albuquerque hospitals enact crisis standards of care during 'unprecedented' time”.
"Currently at UNM today, we're operating at about 140% of our normal operating capacity, and I've had moments where we've approached 150%. This really is an unsustainable and unprecedented level of activity that we've been able to create," Dr. Michael Richards, senior vice president for clinical affairs at UNM Health System, told reporters.
The article makes some oblique references to “Covid pandemic”, making an impression that perhaps the hospitals are overflowing with Covid victims.
The move comes as the hospitals are being stretched to the limit in terms of space and staffing due to increasing COVID-19 hospitalizations and a high volume of patients with acute conditions, officials said.
"Not very good news with hospitalizations," he said, urging people to get vaccinated and booster shots and to follow safe COVID-19 practices. "This is a really serious time."
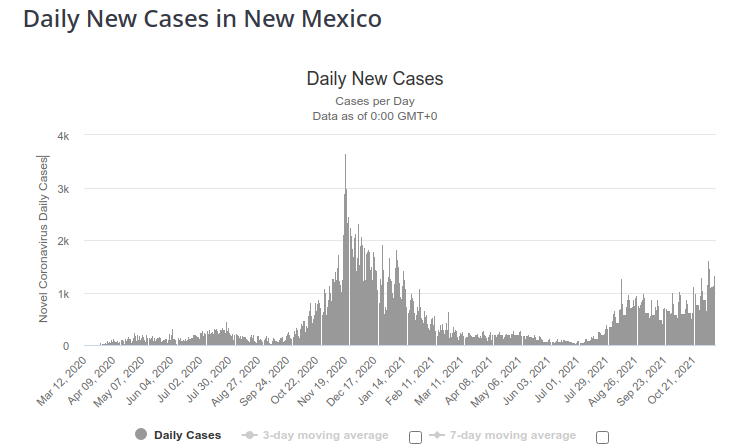
Fortunately, we actually have data that can tell us how many of these beds are occupied with Covid patients. Tha data is right here.
It is a couple of weeks old, but it shows that out of 697 hospital beds and 141 ICU beds, only 43 were occupied with Covid patients. That means that only 5% of beds were taken up by Covid patients.
Since that data was published, nothing big really happened with the Covid pandemic in New Mexico.
So, something happened since two weeks ago, that overflows UNM hospitals, and it is NOT a rapid change in Covid pandemic. What could it be?
Astute readers are probably guessing where this takes us.
So… What is it that is overflowing hospitals? The general sickly vaccinated, or booster victims? I am not sure. Please tell me.
It is, obviously, a pure coincidence that UNM hospitals started overflowing and it coincided with the booster campaign. Boosters are safe and effective. Pfizer tested 12 senior citizens as part of its booster study.
Supportive data from the Phase 1 portion of this study in participants 18 through 55 years of age (N=11) and 65 through 85 years of age (N=12) who had received a 30 µg BNT162b2 prototype vaccine approximately 7 to 9 months after their second dose were also included and consisted of safety data and immunogenicity data evaluating neutralizing antibody titers elicited by the booster dose against the reference strain (wild-type) of SARS-CoV-2 and variants of concern (VOCs).
Nothing to see here. Move along.




My crystal ball....as Pfizer is pushing for the booster for all adults. Being fully vaxxed will be redefined as being no more than 6 months past shot two, and what have been breakthrough cases in the vaccinated will suddenly be classified as unvaccinated cases.
What's hilarious is that on page 4 they state: "Efficacy was not evaluated for Phase 3 BNT162b2 booster group participants." So people are getting boosters under the assumption that waning efficacy of their original doses means they should get another, but they didn't even evaluate this.
Page 5: "Pfizer is requesting approval of the booster dose for use in individuals 16 years of age and older; therefore, safety and effectiveness of the booster dose in individuals 16 and 17 years of age would be based on extrapolation from safety and effectiveness data in adults." Great science, guys. Because of what we saw in the age group comprised of 18-55 with the average age being 38.3 years, we can just extrapolate the data back to the 16-17 year olds. Why stop there? Why not go as far back as the 5 year olds?
But just like all of their FDA briefings, the most important data of all may be found in Table 3 on Page 14. 312 people received the booster shot, and 30 people or nearly 10% of the participants had important protocol deviations resulting in removal from the study. Why is this important? Because it shows a trend. In the original FDA briefing for EUA, there were 311 protocol deviations in the vaccine arm vs 61 in the placebo, or about 5x more. In the 5 - 11 trial, there were 47 protocol deviations in the vaccine arm vs 4 in the placebo, or about 12x more.
Sure, there's a pattern here about there being significantly large amounts of protocol deviations excluding people from the study, but why should that matter? In the article below he lays out how Pfizer may have gotten away with showing that the vaccines were safe. The important parts: 1) if you do not follow the instructions of the study, the trial doctor or BioNTech/Pfizer can remove you from the study, and 2) if you experience symptoms after vaccination, you must tell the study doctor/Pfizer immediately.
So in this scenario, if you got the vaccine, and woke up in the middle of the night with a heart attack and called 911, if you forgot to call the doctor or if you were unconscious and brought to the hospital for treatment, this would give the study doctor/Pfizer the grounds for dismissing you from the trial because you didn't follow instructions.
Are these vaccines safe? Or did they use a clever way to eliminate all people who experienced severe adverse events from the studies?
https://www.nakedcapitalism.com/2021/08/sloppy-pfizer-booster-clinical-trial-consent-form-provides-way-to-exclude-reactions-that-require-emergency-care.html